The Statement
A social media post claims the overwhelming majority of Australian Navy personnel developed severe side effects following COVID-19 vaccinations, reducing the number of healthy staff by half.
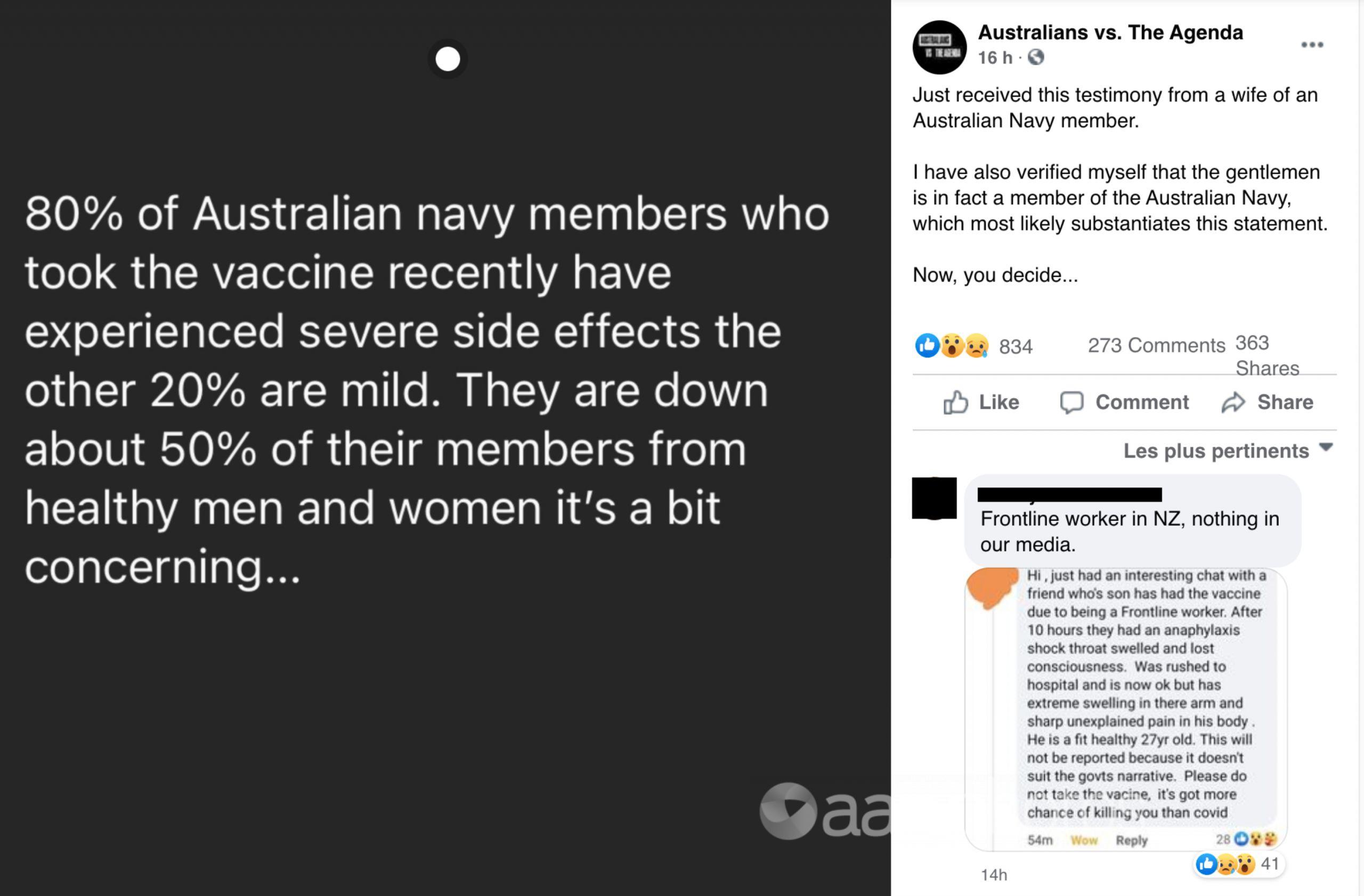
A Facebook post, shared on March 10, claims “80 per cent of Australian navy members who took the vaccine recently have experienced severe side effects, the other 20 per cent are mild”.
The post also claims the Navy is “down about 50 per cent of their members from healthy men and women”.
The post’s caption reads, “Just received this testimony from a wife of an Australian Navy member. I have also verified myself that the gentlemen is in fact a member of the Australian Navy, which most likely substantiates this statement. Now, you decide…”
At the time of writing, the post had more than 30,000 views and more than 480 shares. Another post including similar claims had been shared more than 300 times.
The Analysis
Approximately 500 Australian Defence Force (ADF) members assisting with the country’s frontline pandemic response have received COVID-19 vaccinations, while the Navy crew of HMAS Sydney were also offered inoculations ahead of their deployment to North America.
While some HMAS Sydney crew members who were vaccinated experienced mild side effects, a Department of Defence spokeswoman said none of those reactions were severe – and the ship’s personnel wasn’t reduced to half, as suggested by the post.
Australia began its COVID-19 vaccination rollout in February with quarantine, border, frontline health care and aged care workers among the first to be offered the vaccine. The national health products regulator, the Therapeutic Goods Administration (TGA), provisionally approved both the Pfizer and AstraZeneca COVID-19 vaccines for use.
According to the Department of Defence, approximately 500 ADF members, consisting of Army, Navy and Air Force personnel, have been vaccinated as part of the rollout. The breakdown of numbers from each service was unavailable.
Those ADF members were prioritised to receive the vaccine as they were involved in frontline pandemic response tasks such as quarantine support to the states and territories.
The ship’s company of HMAS Sydney were also offered vaccines ahead of their deployment to North America, “where the prevalence of COVID-19 is high”, a department spokeswoman told AAP FactCheck via email. That was in addition to the 500 other ADF members who received vaccinations.
“In accordance with Department of Health guidelines, members of the ship’s company were encouraged to report to medical personnel if they were feeling unwell after their vaccination. Some members experienced mild side effects, which were resolved shortly after reporting,” the spokeswoman said.
The department initially told AAP FactCheck that “no members of the ship’s company of HMAS Sydney are in hospital” but in a subsequent statement said: “Several members of HMAS Sydney’s crew did present to hospital after hours with mild side effects. They were assessed in the emergency department before being released – they were not admitted to hospital”.
Media reporting at the time also suggested some HMAS Sydney crew members were “admitted to St Vincent’s hospital in Sydney as a precaution”.
A spokesman for St Vincent’s Hospital Sydney said in an email the hospital was “not aware of any such (Navy) patients presenting to our emergency department for treatment in the period you describe (on or before March 11, 2021)”.
In its statement on March 17, the department said the HMAS Sydney sailed to the United States on March 11 with a full crew and “no members of the ship’s company failed to deploy as a result of taking the COVID-19 vaccine”.
“Social media commentary from 10 March 2021 asserting that members of HMAS Sydney who received the COVID-19 vaccine had experienced adverse reactions and were hospitalised in intensive care – and that 80 per cent of the Ship’s company were affected – is not true.”
Various images of the ship’s departure on that day were shared on social media, while several days later posts showed the vessel in Suva, Fiji.
On March 11, Australia’s Chief Medical Officer, Paul Kelly, appeared before a Senate committee on COVID-19 and was questioned about the reporting of adverse reactions related to the vaccine.
Prof Kelly said he would be notified of every serious adverse event relating to COVID-19 vaccines, and there had only been a very small number Australia-wide.
“We have seen three cases now of anaphylactic reactions – severe allergic reactions – each of which have been handled very expertly and quickly by the clinical teams on the ground, with no ongoing adverse effects,” he said.
The TGA said on March 1 that early COVID-19 vaccine data showed around one-third of people who responded to a survey for Australia’s vaccine surveillance program reported at least one expected side effect, most commonly soreness at the injection site, headaches and fatigue.
Reports of adverse events were in line with normal expectations for any vaccine, it said. Most were mild and lasted only one or two days.
As of March 14, Australia had administered more than 164,000 doses of COVID-19 vaccines, according to the Department of Health. On March 17, Queensland reported four cases of anaphylaxis in people who received the AstraZeneca vaccine. These cases were referred to the TGA for investigation.

The Verdict
There is no credible evidence to support the claim that 80 per cent of Navy personnel experienced severe side effects after receiving COVID-19 vaccinations.
According to the Department of Defence, some HMAS Sydney members experienced mild side effects after receiving COVID-19 vaccinations. Several attended a local hospital after hours but none required admission, and the ship departed with a full crew on March 11.
False – Content that has no basis in fact.
AAP FactCheck is an accredited member of the International Fact-Checking Network. To keep up with our latest fact checks, follow us on Facebook and Twitter.
All information, text and images included on the AAP Websites is for personal use only and may not be re-written, copied, re-sold or re-distributed, framed, linked, shared onto social media or otherwise used whether for compensation of any kind or not, unless you have the prior written permission of AAP. For more information, please refer to our standard terms and conditions.

















