AAP FACTCHECK – An unnamed nurse claims a “genetic vaccine” has been secretly added to Australia’s childhood vaccine schedule.
This is false. The vaccine in question doesn’t contain DNA and its addition to the program was publicly announced.
The claim appears in a Facebook post featuring a screenshot of an email, purportedly from a registered nurse working in an Australian GP practice.
The nurse claims the vaccine Vaxelis was secretly added to the National Immunisation Program (NIP) Schedule, a series of immunisations from birth through to adulthood.
“So….I checked out the official Product Information for Vaxelis, and found that it’s s [sic] a ribosomal DNA vaccine,” the post’s caption reads.
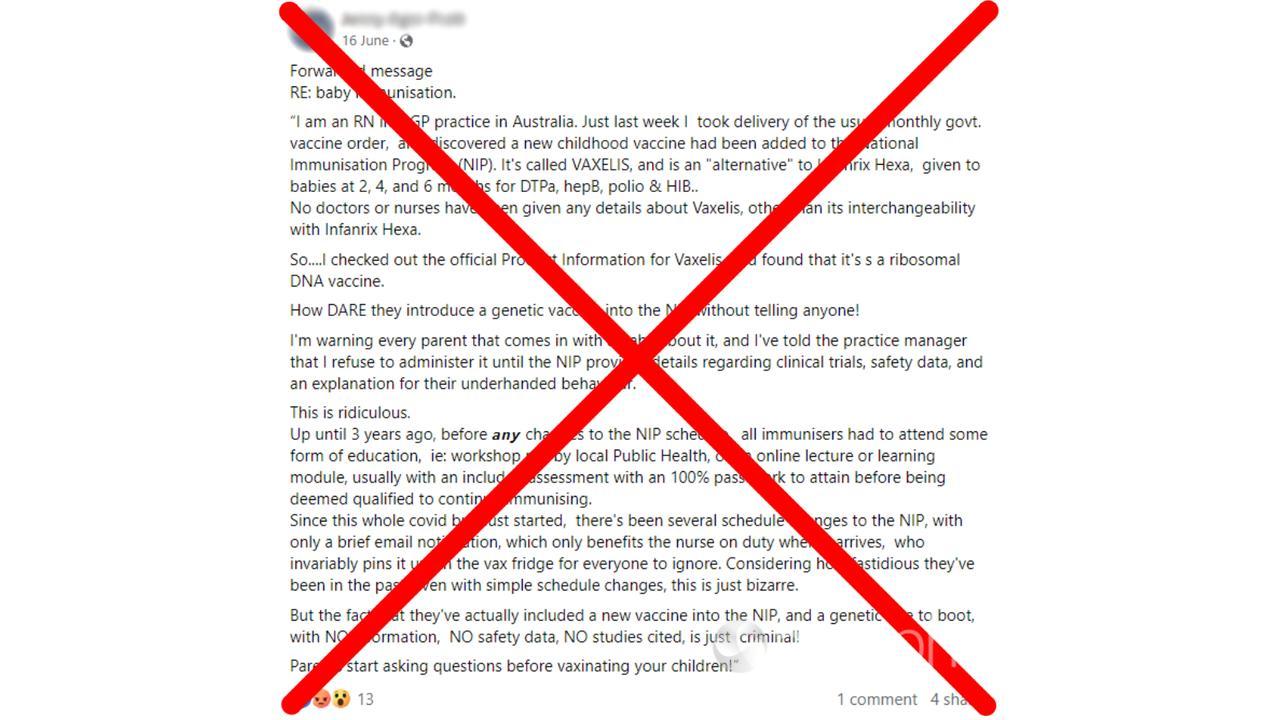
“How DARE they introduce a genetic vaccine into the NIP without telling anyone!”
However, experts say Vaxelis does not contain any DNA.
Infectious diseases physician Allen Cheng told AAP FactCheck the hepatitis B protein used in the new vaccine is made using a “recombinant DNA” process, meaning it was created artificially by combining DNA from different sources, but the vaccine itself does not contain DNA.
Ribosomal DNA and recombinant DNA are both shortened to “rDNA”, which could be what triggered the false claim.
Recombinant DNA is a common technique in creating protein vaccines, Murdoch University immunology professor Cassandra Berry told AAP Factcheck, while infectious disease dynamics modeller James Wood said protein vaccines have been used to treat hepatitis B for more than 30 years.
Prof Berry added that using the term “ribosomal DNA” doesn’t make sense for protein vaccines.
There is no reason to suspect Vaxelis would pose any greater health risk than the original (and still recommended) vaccine Infanrix hexa.
According to the National Centre for Immunisation Research and Surveillance’s policy director Helen Quinn, the manner in which Vaxelis was announced is typical for vaccines in Australia.
Dr Quinn also confirmed: “There is no DNA or RNA in this vaccine.”
The new vaccine was announced on the Department of Health and Aged Care’s website on June 30, 2023, and the site also has a timeline showing it was approved in April 2022 after several months in development.
The Australian Technical Advisory Group on Immunisation also conducted an evidence review prior to introducing the vaccine.
The ABC’s has also debunked the claim.
The Verdict
False – The claim is inaccurate.
AAP FactCheck is an accredited member of the International Fact-Checking Network. To keep up with our latest fact checks, follow us on Facebook, Twitter and Instagram.
All information, text and images included on the AAP Websites is for personal use only and may not be re-written, copied, re-sold or re-distributed, framed, linked, shared onto social media or otherwise used whether for compensation of any kind or not, unless you have the prior written permission of AAP. For more information, please refer to our standard terms and conditions.


















