Showcasing at EURETINA 2024: ZEISS will highlight new surgical innovations and artificial intelligence (AI) tools for retinal patient care, helping doctors diagnose and treat patients efficiently and effectively:
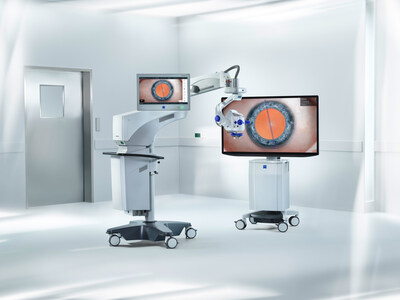
- THE FUTURE OF VITREORETINAL SURGERY: Demonstrating the ZEISS ARTEVO 850 3D digital visualization system, Single-use Lenses for ZEISS RESIGHT, and the EVA NEXUS surgical system from DORC.
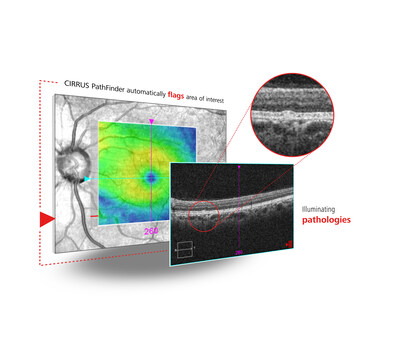
- AI ADVANCEMENTS FOR RETINA PATIENTS: CIRRUS PathFinder introduces a paradigm shift in the ability to streamline OCT data review, including large amounts of OCT scans flagging macular B-scans that may require closer review.
- SIMPLIFIED SURGICAL VIDEO MANAGEMENT: Showing a digital application for experiencing 3D videos and more on the Apple Vision Pro, offering surgery video review, analysis, and sharing post-surgery.
JENA, Germany, Sept. 12, 2024 /PRNewswire/ — ZEISS Medical Technology will showcase digital innovations and its latest 3D visualization technology as part of the ZEISS Retina Workflow at the European Society of Retina Specialists (EURETINA) conference. The new solutions within the ZEISS Medical Ecosystem help improve the diagnosis and treatment of retinal patients using the latest integrated, digital technologies.
“At EURETINA this year, ZEISS will demonstrate our latest surgical innovations and digital tools designed to help retinal surgeons deliver diagnoses and treatment plans efficiently and with good patient outcomes,” said Euan S. Thomson, Ph.D., President of the Ophthalmology Strategic Business Unit and Head of the Digital Business Unit at ZEISS Medical Technology. “We’re excited to showcase our expanding ZEISS Retina Workflow, now including DORC’s EVA NEXUS surgical system which complements our overall ophthalmic surgery portfolio by delivering exceptional efficiency, improved stability and higher responsiveness during surgery.”
The future of 3D digital visualization in ophthalmic surgery
ZEISS will showcase advanced surgical technologies that are setting the pace in 3D digital visualization in ophthalmic surgery. The ARTEVO® 850 3D digital ophthalmic microscope, used in both anterior and posterior surgical cases, offers customizable 3D digital visualization with true color imaging allowing the surgical image to be displayed in high fidelity as well as an expanded depth of field (DoF), enabling surgeons to increase the depth of field by nearly 60 percent.1 The ZEISS ARTEVO 850 offers digital integration into the surgical workflow for seamless data transfer and a redesigned CALLISTO eye® user interface for intuitive navigation and productivity. The integrated intraoperative Optical Coherence Tomography (OCT) with real-time dimension for viewing transparent structures of the eye during surgery enables confident decision making during vitreoretinal surgery. Additionally, the Digital Color Assistant (DCA) accentuates anatomical details with digital color coding at a click.
Professor Siegfried Priglinger, Head and Chair of the Eye Hospital at the Ludwig-Maximilian-University Munich (Germany), explains, “When working with this 3D heads-up system for anterior and posterior segment surgery, including intraoperative OCT, I have experienced the big advantage of preoperative diagnostics and intraoperative work now being connected together. I think this is something you get really comfortable with because you always have it with you in the OR.”
Additionally, the Single-use Lenses for the RESIGHT® fundus viewing system from ZEISS provide retinal surgeons with a clear and uncompromised view for every surgical procedure in the posterior segment, while also offering all the advantages of disposable lenses. Says Professor Peter Stalmans, a vitreoretinal specialist at UZ Leuven (Belgium): “With the Single-use Lenses for ZEISS RESIGHT, I can operate in a way that is safer for patients’ eyes. I can reduce my endoillumination to 10 percent without compromising the view and with the new ULTRA WIDE-ANGLE LENS I can remove all the vitreous up to the ora serrata without the need to indent a patient’s eye.”
DORC, now a ZEISS company, will showcase the EVA NEXUS surgical system as part of the ZEISS Retina Workflow, complementing ZEISS’s ophthalmic surgery portfolio by enabling more responsive and precise control for the surgeon. As one of the market’s most advanced dual-function, vitreoretinal and cataract surgical systems, the EVA NEXUS platform is the core of a comprehensive vitreoretinal surgery portfolio, comprising a full range of instruments, dyes, and tamponades for a best-in-class solution for vitreoretinal (VR) procedures.
AI-based tools streamline retinal diagnostics and revolutionize the use of surgery videos
AI tools from ZEISS support streamlined workflows for ophthalmologists by analyzing data at scale, enabling data-driven decisions, and facilitating early detection of diseases. ZEISS currently offers numerous AI-powered solutions for ophthalmology, including automated analysis of diagnostic test data, anomaly and disease detection, classification of disease stage, assessment of change, prediction of future progression, evaluation of data quality, artifact identification, and image quality enhancement.
The company will show CIRRUS® PathFinder™, a deep learning AI-based support tool that enables more efficient review of OCT data. Designed to complement the ZEISS Retina Workflow, CIRRUS PathFinder2 streamlines the review of large volumes of OCT data by identifying scans that may require closer review. ZEISS CIRRUS PathFinder’s AI-enabled detection, which is seamlessly integrated into the ZEISS CIRRUS OCT interface, can identify OCT B-scans with macular findings. It also provides insights to optimize treatment paths, improve outcomes, and deliver personalized patient care. ZEISS CIRRUS PathFinder will be available in selected markets, subject to local regulatory clearances, and is currently pending CE mark and FDA clearance.
At EURETINA, ZEISS will also demonstrate a new product for ophthalmologists to review surgery videos pre-recorded with ZEISS surgical microscopes. The ZEISS Surgery Optimizer3 is an AI-powered application that revolutionizes the use of surgery videos. It simplifies the process of exporting videos from ZEISS CALLISTO eye, allows access to surgery videos via web browser or mobile device4, and facilitates documentation and review in an automatically arranged case library. Based on the Surgery Optimizer, a new spatial computing app will be demonstrated as an exclusive experience on Apple Vision Pro. Using an Apple Vision Pro, surgeons can review 2D or 3D surgery videos, scans of the eye, patient information and much more simultaneously, providing a glimpse into the future of ophthalmology.5
ZEISS will showcase its new ZEISS Retina Workflow innovations at the EURETINA conference in Barcelona, Spain, from September 19-22, 2024, at the International Barcelona Convention Center, Floor P1, Booth E3.
1 Compared to ARTEVO 800, data on file.
2 PathFinder works on all current CIRRUS devices: 500, 5000, 6000, pending local regulatory clearances.
3 ZEISS Surgery Optimizer is a non-medical device and not intended for diagnosing, treatment, or prophylactic activities.
4 Only works on iPhone or iPad. iPhone and iPad are trademarks of Apple Inc. iOS is a trademark of Cisco technology, Inc.
5 The concept on Apple Vision Pro only allows the review of pre-selected clinical cases.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development. The statements of the healthcare professionals reflect only their personal opinions and experiences and do not necessarily reflect the opinion of any institution that they are affiliated with. The healthcare professionals alone are responsible for the content of their experience reported and any potential resulting infringements. Carl Zeiss Meditec AG and its affiliates to not have clinical evidence supporting the opinions and statements of the health care professionals nor accept any responsibility or liability of the healthcare professionals’ content.
The healthcare professionals have a contractual or other financial relationship with Carl Zeiss Meditec AG and its affiliates and have received financial support.
Contact for investors:
Sebastian Frericks
Head of Group Finance and Investor Relations
Carl Zeiss Meditec AG
Phone: +49 3641 220 116
Mail: investors.med@zeiss.com
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world’s leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 4,823 employees worldwide, the Group generated revenue of €2,089.3m in fiscal year 2022/23 (to 30 September).
The Group’s head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company’s presence in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG’s shares are in free float. The remaining approx. 59 percent are held by Carl Zeiss AG, one of the world’s leading groups in the optical and optoelectronic industries.
For further information visit: www.zeiss.com/med



SOURCE Carl Zeiss Meditec AG


















